Patient consent is a fundamental aspect of ethical and legal medical practice, particularly in cosmetic gynecology, where procedures are elective and often emotionally charged. Proper consent protects patient autonomy, promotes transparency, and helps practitioners navigate legal and ethical responsibilities.

In the U.S., informed consent follows state laws, federal regulations such as HIPAA and the Common Rule, and professional guidelines. Failure to obtain proper consent can cause legal disputes. In this guide, we’ll discuss legal frameworks, ethical considerations, best practices, and challenges practitioners may face.
What is Informed Consent?
Informed consent is the process where a patient voluntarily agrees to a medical procedure, treatment, or research participation after receiving sufficient information. This process involves an open discussion between the practitioner and the patient, letting the patient understand the nature of the procedure, the risks and benefits, alternatives, and the consequences of declining treatment.
Components of a Valid Informed Consent
A legally and ethically valid informed consent process includes five essential elements:
- Disclosure: Explanation of the procedure, risks, benefits, and alternatives.
- Comprehension: The patient understands the provided information.
- Competence: The patient has the mental capacity to decide.
- Voluntariness: Consent is given freely, without pressure.
- Documentation: Written or verbal acknowledgment of consent is recorded.
This means that for consent to be valid, the patient must have the mental capacity to make decisions, understand the provided information, and make their choice without coercion.
Step-by-Step Process for Obtaining Consent
Practitioners in cosmetic gynecology can enhance the informed consent process by following structured steps that address both medical and aesthetic considerations.
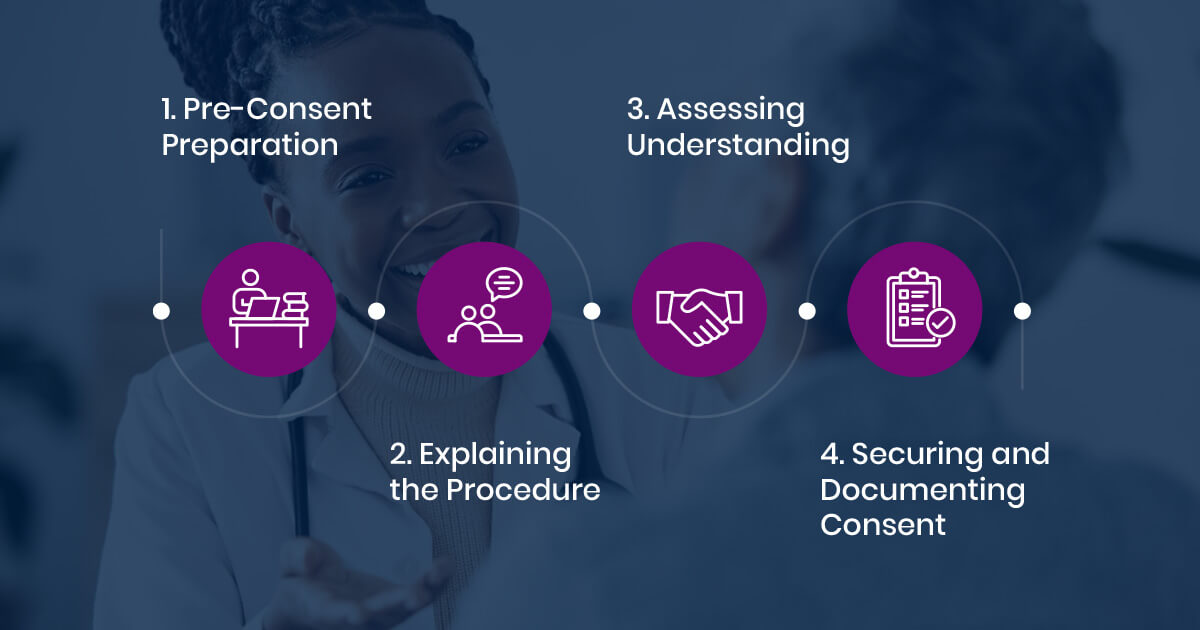
1. Pre-Consent Preparation
Practitioners should gather necessary information before discussing consent with a patient. It is important to assess the patient’s literacy level and provide easy-to-understand materials. If the patient speaks a different language, an interpreter should be arranged to facilitate clear communication.
2. Explaining the Procedure
When explaining the procedure, practitioners should clearly describe the diagnosis, treatment options, benefits, and risks. Simple language should be used to avoid confusion, and visual aids can help improve understanding.
3. Assessing Understanding
To confirm the patient comprehends the information, the “Teach-Back” Method can be used. This involves asking the patient to summarize key points of the discussion. Practitioners should also observe for any signs of confusion or distress and address them as needed.
4. Securing and Documenting Consent
Once understanding has been confirmed, practitioners should secure consent using standardized forms. Written consent should be obtained for major procedures, while verbal consent should be documented in medical records for minor procedures.3
Procedure-Specific Consent Considerations
Cosmetic gynecological procedures involve unique consent elements beyond general surgical risks.
- Labiaplasty: Patients should be informed about potential risks such as scarring, asymmetry, and changes in sensation. Healing timelines and revision possibilities should also be discussed.
- Vaginoplasty: Consent should include potential risks related to functional outcomes, sensation changes, and post-surgical care.
- Non-Surgical Procedures (Laser Therapy, PRP Injections, Radiofrequency Tightening): Patients should be made aware of discomfort levels, the need for multiple sessions, and limitations in achieving desired results.
Managing Patient Expectations
One of the most significant aspects of informed consent in cosmetic gynecology is ensuring that patients have realistic expectations. Practitioners should:
- Provide clear explanations of expected results, including limitations.
- Emphasize that aesthetic outcomes vary based on anatomy and healing response.
- Discuss the possibility of revision procedures.
- Obtain patient acknowledgment that they understand the nature of expected results.
Legal and Ethical Frameworks in the U.S.

The Legal Basis of Consent
The legal framework for patient consent varies by state but follows federal regulations and case law precedents:
| Legal Standard | Description |
| Informed Consent Law | Most states follow Canterbury v. Spence (1972), which requires practitioners to disclose material risks.1 |
| Common Rule (45 CFR 46) | Governs informed consent in human subjects research. |
| HIPAA (1996) | Regulates consent for data sharing in healthcare settings. |
| Federal Policy for the Protection of Human Subjects (2018 revision) | Strengthens consent requirements for research participation.2 |
Psychological Factors Influencing Consent in Cosmetic Gynecology
Psychological factors are important in informed consent for cosmetic gynecology. Emotional motivations and external influences can affect patient decisions, such as the following:
- Body Dysmorphia and Psychological Distress: Studies indicate that individuals with body dysmorphic disorder (BDD) are more likely to seek cosmetic procedures but may have lower satisfaction post-surgery. Psychological risk factors such as depression, perfectionism, and self-criticism are linked to negative post-surgical experiences.
- Social Pressure and Partner Influence: Societal beauty standards, media influence, and peer pressure contribute to a patient’s decision to undergo cosmetic gynecology procedures. Some patients may also experience pressure from partners, raising ethical concerns about autonomy in decision-making.
- Unrealistic Expectations and Post-Surgical Adjustment: Patients who expect surgery to drastically improve their relationships or emotional well-being may be at risk for dissatisfaction. Informed consent discussions should address realistic outcomes and emotional adjustments.
Case Study: Unauthorized Procedure and Patient Autonomy

In Brooke Shields Is Not Allowed to Get Old, Brooke Shields revealed that a surgeon performed an additional procedure without her consent.4 Seeking a labial reduction for discomfort, she later learned the surgeon had also performed vaginal tightening without her approval, presenting it as a favor. This unauthorized intervention left her in shock and raised serious ethical concerns.
Shields later recognized the clear violation of her rights but hesitated to take legal action due to media scrutiny. Her experience highlights the importance of documented consent in cosmetic gynecology, reinforcing the ethical duty of practitioners to fully inform patients and respect their autonomy.
Implications of Not Obtaining Consent
There are significant legal, professional, and ethical consequences that come with the failure to obtain informed consent:
Legal and Professional Risks
- Medical Malpractice Claims – Patients who suffer harm from a procedure they did not consent to may file lawsuits, leading to financial liability for healthcare providers.
- Disciplinary Actions – Medical boards can impose penalties, including suspension or revocation of a provider’s license, for failing to follow consent protocols.
- Increased Liability – Without proper consent documentation, healthcare providers face greater exposure to negligence claims and legal disputes.
Ethical and Patient Trust Concerns
- Erosion of Trust – Patients who feel deceived or uninformed may lose confidence in their provider, affecting long-term care relationships.
- Violation of Autonomy – Informed consent respects a patient’s right to make decisions based on their values and preferences. Ignoring this right undermines ethical medical practice.
- Weakened Risk Management – Clear documentation of consent discussions serves as a legal safeguard. Without it, providers have little defense in case of disputes.
Conclusion

Informed consent in cosmetic gynecology is both a legal formality and a process that fosters trust and minimizes risks. Practitioners should focus on clear communication, voluntary decision-making, and proper documentation, while also considering psychological factors and managing patient expectations effectively.
How We Can Help
At the American Board of Cosmetic Gynecology, we offer certification programs that help practitioners strengthen their understanding of ethical and legal standards, including informed consent. Our training supports compliance with regulations while enhancing patient care. Get certified with us today!
References
- C. Manthous et al. “Informed consent for medical procedures: local and national practices..” Chest, 124 5 (2003): 1978-84 . https://doi.org/10.1378/CHEST.124.5.1978.
- M. Cho et al. “Attitudes Toward Risk and Informed Consent for Research on Medical Practices.” Annals of Internal Medicine, 162 (2015): 690-696. https://doi.org/10.7326/M15-0166.
- A. Jibawi et al. “Good consent practice.” Current Surgical Guidelines (2009). https://doi.org/10.1093/med/9780198794769.003.0006.
- McNeil, Liz. “Brooke Shields Got a ‘Bonus’ Labia Rejuvenation Without Her Consent: ‘Why Can’t Everybody Just Leave My Vagina Alone’ (Exclusive).” People.com, 10 Jan. 2025, people.com/brooke-shields-cosmetic-surgery-without-consent-new-book-excerpt-8770612


